1) Write the formula of both reactants.
Iron (III) sulfide = Fe2S3
Sodium phosphate = Na3PO4
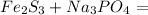
2) Expected products
Iron(III) phosphate: FePO4
Sodium sulfide: Na2S
3) Complete equation (unbalanced)
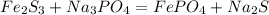
4) Balance Iron (Fe)
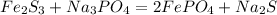
5) Balance Sodium (Na)
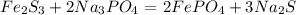
After balancing Iron and Sodium the chemical equation is completely balanced.
6) Word equation
Iron (III) sulfide + Sodium phosphate = Iron(III) phosphate + Sodium phosphate