Step 1
2NOCl(g) ⇋ 2NO(g) + Cl2(g) (balanced)
Initial 1.3 M 0 0
Reacts -2x 2x x
Equilibrium 1.3-2x 2x x
Equilibrium 0.44 M 2x0.43= 0.86M 0.43M
Concentrations:
Volume of the chamber = 2.50 L
[NOCl] initial = 3.25 moles/2.50 L = 1.3 M
After equilibrium was reached, 1.10 moles of NOCl remained. Therefore,
1.3 - 2x = 1.10 moles/2.50 L = 0.44 M
(1.3 - 0.44)/2 = x = 0.43 M
------------------------
Step 2
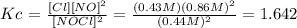
Answer: Kc = 1.642