The equation is balanced since the number of atoms of the elements on each side of the reaction is equal. So we can continue with the calculations.
We have that the Al to O2 ratio is 4/3, we know this from the stoichiometry of the reaction by seeing the coefficients that accompany the Al and O2 molecules.
So the moles of Al will be:
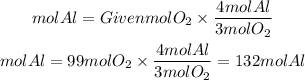
If you have 99 moles of O2 you need 132 moles of Al to use it all.
Answer: 132mol Al