Answer:
3.6%
Explanation:
Given the measurements recorded by Bob below:
• Recorded Volume = 14.3 mL
,
• Actual Volume = 13.8 mL
The goal is to find the percent error to the nearest tenth of a percent.
The formula for percentage error is given below:
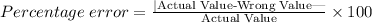
Substitute the measurements:
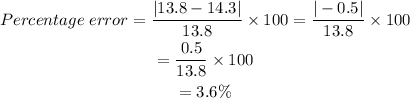
The percent error of Bob's measurement is 3.6% (to the nearest tenth of a percent).