The percent composition of an element in a compound can be found by the following formula:
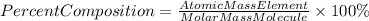
Therefore, we must first find the weight of the lead(II) nitride molecule. The molecular formula of lead (Il) nitride is Pb3N2, so the molar mass of the element will be:
Molar Mass Pb3N2= Atomic Mass Pb x 3 + Atomic Mass N x 2
the values of the atomic mass of the elements are found in the periodic table.
Atomic Mass Pb=207.2u
Atomic Mass N=14.0067u
So, the molar mass of Pb3N2 will be:
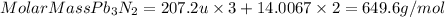
The percent composition of nitrogen in Pb3N2 will be:
![\begin{gathered} PercentCompositionN_2=(14.0067*2)/(649.6)*100\% \\ PercentCompos\imaginaryI t\imaginaryI onN_2=(28.0134)/(649.6)*100\operatorname{\%} \\ PercentCompos\imaginaryI t\imaginaryI onN_2=4.31\% \end{gathered}]()
Answer: The percent composition of nitrogen in a compound of lead (Il) nitride is 4.31%