If we add x liters of the pure acid, we must have 90-x liters of 10% acid solution.
The equation that represents the situation:
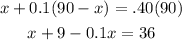
Solve for x to determine the liters of pure acid:
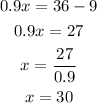
30 liters of pure acid need to be used to make the solution.
Then, 90-x would be the amount of the 10% acid solution:
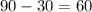
60 Liters of 10% acid solution is needed to make the solution.