The equation is balanced and we see that two moles of hydrogen produce two moles of water. The relationship is 1 to 1 since the same amount of H2 that reacts corresponds to the moles of water that are produced.
Now, we determine the number of moles of H2 present using the molar mass of hydrogen equal to 2.01568 g/mol.
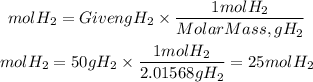
Since the ratio is 1 to 1, 25 moles of H2 will produce the same number of moles, so the answer is:
50 grams of hydrogen could produce 25 moles of water