The balanced equation for decane combustion is:
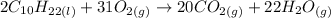
That is the balanced equation.
To calculate the volume of carbon dioxide, use the mass of decane and convert it to moles (use the molecular weight of decane, 142g/mol), then use the ration of the coefficients of carbon dioxide to decane to find the number of moles of CO2 produced:
![0.400\operatorname{kg}\cdot\frac{1000g}{\operatorname{kg}}\cdot(1mol)/(142g)=2.82mol]()