Nitrogen is an element from group 15 and the second period. Its atomic number is 7, which means it has 7protons and 7 electrons in the neutral state.
Let's use the general electron configuration rule to figure out the configuration for Nitrogen.
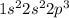
If we sum up the exponents, which refer to the number of electrons in each sublevel, we get to the number of electrons, which is 7 for Nitrogen.
To answer this question, we must consider only levels instead of sublevels. So on level 1 there are 2 electrons and on level 2 there are 2 + 3 = 5 electrons. Therefore we get to the answer:
C. 2, 5