Answer:
72.2g of oxygen gas are needed.
Step-by-step explanation:
1st) It is necessary to balance the chemical reaction:
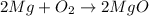
Now we know that 2 moles of Mg will react with 1 mol of oxygen gas to produce 2 moles of MgO.
2nd) With the molar mass of oxygen gas, we can convert moles into grams, and calculate the grams of oxygen gas needed to completely burn 4.51 moles of Mg:
- O2 molar mass: 32g/mol
From the stoichiometry of the reaction and the molar mass, we know that 32g of oxygen gas need 2 moles of Mg to react properly. Now, with a mathematical rule of three we can calculate the grams of O2 gas needed:
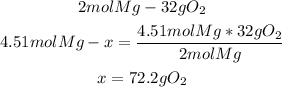
Finally, 72.2g of oxygen gas are needed.