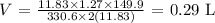Answer:
The final volume of the gas is 0.29 L
Step-by-step explanation:
Here, we want to get the final volume of a given mass of gas
From the question, we are given the initial condition values, and we want to get the final volume
The gas law to use is the general gas law
We have the mathematical representation as follows:
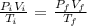
While i represents the intial values, the f values represents the final values
Let us get the values as given in the question
Initial values:
Pressure = 11.83 kPa
Volume = 1.27 L
Temperature = 330.6 K
Final values;
Pressure = 2(11.83) kPa : The pressure value was doubled
Volume = ?
Temperature = 149.9 K
We proceed to substitute these values into the equation written above
We have this as follows:
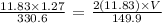
Finally, we get the volume value as follows: