The balanced chemical equation b. 2 C₄H₁₀ + 13 O₂ ⇒ 8 CO₂ + 10 H₂O
Further explanation
Given
Butane (C₄H₁₀)
Required
Balanced equation
Solution
Formula
Hydrocarbon combustion reactions (specifically alkanes)
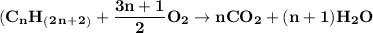
In the combustion process, the compound in the reactants is Oxygen (O₂)
If the oxygen needed for combustion is sufficient (or excess) then the combustion results are in the form of CO₂ and H₂O, but if not enough, CO and H₂O will be obtained.
The only answer that contains O₂ in the reactants is option B