Step 1
Aluminum chloride has the next chemical formula:
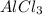
--------------------
Step 2
The number of moles (n) is calculated as follows:
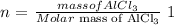
-------------------
Step 3
Information provided:
4.36 moles of AlCl3
---------------
Information needed:
The molar mass of AlCl3 = 133 g/mol
-------------------
Step 4
The mass is found from (1):
n x molar mass of AlCl3 = mass of AlCl3
4.36 moles x 133 g/mol = 580 g approx.
Answer: 580 g of AlCl3