Answer:
The longest wavelength of radiation is 494.6 nm that will possess the energy to break the bond.
Step-by-step explanation:
Energy required to break 1 mol of Cl-Cl bond = 242 kJ =242000 J
1kJ = 1000 J
1 mol =
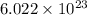 atoms/particles/ molecules
atoms/particles/ molecules
Energy required to break 1 of Cl-Cl bond = E

Wave length of the radiation with energy E.

h = Planck's constant
c = speed of the light


The longest wavelength of radiation is 494.6 nm that will possess the energy to break the bond.