Answer:
1:1.
Step-by-step explanation:
Hello!
In this case, since magnesium, in the form that is able to react with sulfur, has an oxidation state of 2+ and sulfur 2-, because they need to be different in order to form a suitable compound; due to that equality in their oxidation states, we expect they react in a 1:1 mole ratio, according to the following example:
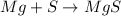
Best regards!