ANSWER
The number of moles of KMnO4 is 0.0350 moles
Step-by-step explanation
Given that:
The mass of KMnO4 is 5.53 g
To find the number of moles of KMnO4, follow the steps below
Step 1: Write the formular for calculating the number of moles
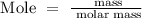
Step 2: Find the molar mass of KMnO4
Recall, that the molar mass of KMnO4 is 158.034 g/mol
Step 3: Substitute the given data into the formula in step 1
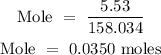
Therefore, the number of moles of KMnO4 is 0.0350 moles