ANSWER
The number of atoms of phosphorus is 1.668 x 10^24 atoms
Step-by-step explanation
Given that;
The mass of phosphorus is 85.64 grams
Follow the steps below to find the atoms of phosphorus
Step 1; Find the moles of phosphorus using the formula below
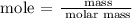
Recall, that the unit mass of phosphorus is 30.97u
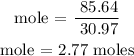
Step 2; Find the number of atoms of phosphorus using the formula below
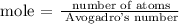
Note, that Avogadro's number is 6.02 x 10^23

Therefore, the number of atoms of phosphorus is 1.668 x 10^24 atoms