We are given the following balanced equation:
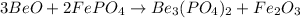
We want to calculate the mass of Fe2O3 that will be produced from 38 g BeO.
We first need to calculate the number of moles of BeO
n = m/M where n is the number of moles, m is the mass and M is the molar mass. Molar mass of BeO = 25,01158 g/mol
n = 38 g/25,01158 g/mol
n = 1.519 mol
Now that we have the number of moles of BeO, we can use the stoichiometry to find the number of moles of Fe2O3.
The molar ratio between BeO and Fe2O3 is 3:1
Therefore the number of moles of Fe2O3 = 1.519 x (1/3) = 0.506 mol
Now that we do have the number of moles of Fe2O3, we can find the mass by re-arranging the above equation: The molar mass of Fe2O3 = 159,69 g/mol
n = m/M
m = n x M
m = 0.506 mol x 159,69 g/mol
m = 80.87 g