To solve this problem we need to know first how many mols of water we have. To do that we use the Ideal gas law with the that provided:
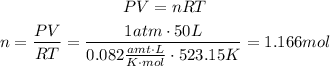
according to the chemical equation we know that 3 mols of water react with 2 mols of iron, we can use this information as a conversion factor to know how many mols of iron react with 1.166 mols of water:
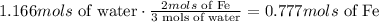
and last we have to convert those mols into mass. To do that we use the molar mass of Iron 55.845g/mol:
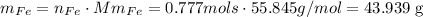
the mas of Iron is 43.939g