Explanations
4) According to the question, we are to determine which type of reaction are the given reaction by using the given reactions in the table.
The reaction between lead (II) hydroxide and hydrochloric acid to produce lead(II) chloride and water is an acid-base reaction that involves the reaction between an acid and base to produce salt and water
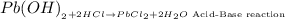
For the second reaction:
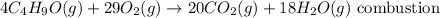
This reaction is a combustion reaction since it occurs under oxygen to produce carbon dioxide and water.
For the third reaction:
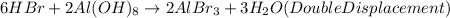
This is a double displacement reaction since it involves the reaction between two compounds to produce another two compounds.
For the fourth reaction given as:
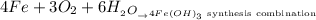
This is a synthesis combination since two compounds react to form a single new compound
For the fifth reaction:
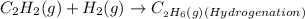
This is a hydrogenation reaction since it involves the addition of hydrogen to the given compound to produce a new compound.