Answer
The percent ionization of formic acid is 3.69%.
Step-by-step explanation
Given:
Ka of formic acid = 1.77 x 10⁻⁴
Molarity of formic acid = 0.125 M
What to find:
The percent ionization of formic acid.
Step-by-step solution:
The dissociation of formic acid is given as:
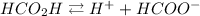
The acid dissociation constant (Ka) for formic acid is given as:
![K_a=([H^+][HCOO^-])/([HCO_2H])](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/njrwe2512rdzb5xy9q0t.png)
Substituting the concentration of the ions and the acid into the acid dissociation constant above:
![\begin{gathered} 1.77*10^(-4)=([x][x])/([0.125-x]) \\ \\ x=4.61*10^(-3) \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/k8yh8fjqjxny9bj120a7.png)
The hydrogen ion concentration in the solution = 0.00461 M.
The percent ionization of the formic acid can be calculated using the formula below:
![Percent\text{ }ionization=([H^+])/([HCO_2H])*100\%](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/830qeu5r3az9nydezpzs.png)
Putting [H⁺] = 0.00461 M and [HCO₂H] = 0.125 M into the formula
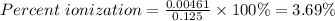
The percent ionization of formic acid is 3.69%.