1. First, we have to put the products of the reaction:
![\begin{gathered} CaBr_2\text{ + NaOH }\rightarrow\text{ Ca\lparen OH\rparen}_2\text{ + NaBr} \\ \\ If\text{ we notice, we have to balance in this order: } \\ \\ 1.\text{ Ca: 1 atom}\rightarrow\text{ 1 atom Balanced} \\ \\ 2.\text{ Br: 2 atoms }\rightarrow\text{ 1 atom. Then: } \\ \\ CaBr_2+\text{ NaOH}\operatorname{\rightarrow}\text{ Ca}\operatorname{\lparen}\text{OH}\operatorname{\rparen}_2\text{ + 2NaBr} \\ \\ 3.\text{ Na: 1 atom }\rightarrow\text{ 2 atoms. Then: } \\ \\ CaBr_2+\text{ 2NaOH}\operatorname{\rightarrow}\text{ Ca}\operatorname{\lparen}\text{OH}\operatorname{\rparen}_2+\text{ 2NaBr} \\ \\ 4.\text{ H: 2 atoms }\rightarrow\text{ 2 atoms Balanced} \\ \\ 5.\text{ O: 2 atoms }\rightarrow\text{ 2 atoms Balanced. } \end{gathered}]()
Then, the last equation is already balanced.
2. If we have the correct balanced equation, now we have to calculate the molecular weight of the involved substances:
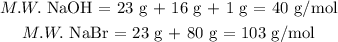
Now, we can calculate the theoretical amount of NaBr that is produced, assuming that we have an excess of CaBr2:
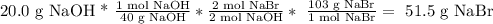
Then, the produced amount of NaBr is 51.5 g.