We have the same number of atoms of each element on each side of the reaction. This means that the equation is balanced and we can continue.
They give us the moles of Pb(NO3)2 that can react. We must find the ratio PbBr2 to Pb(NO3)2, we do this by looking at the stoichiometry of the reaction. We see the coefficients that are before the molecules. We have that the ratio PbBr2 to Pb(NO3)2 is 1/1.
This means that the same moles of Pb(NO3)2 that react are the same moles of PbBr2 that are produced. In general, the following equation applies:
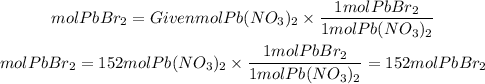
If you have 152 moles of Pb(NO3)2 you can produce 152 moles of PbBr2 .
Answer: 152 moles of PbBr2