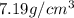Answer: The density of metal X is
Step-by-step explanation:
We are given:
Atomic radius of metal X =
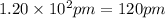
To calculate the edge length, we use the relation between the radius and edge length for FCC lattice:
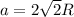
Putting values in above equation, we get:
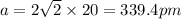
To calculate the density of metal, we use the equation:
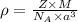
where,
 = density
= density
Z = number of atom in unit cell = 4 (FCC)
M = atomic mass of metal = 42.3 g/mol
 = Avogadro's number =
= Avogadro's number =
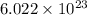
a = edge length of unit cell =
 (Conversion factor:
(Conversion factor:
 )
)
Putting values in above equation, we get:
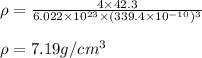
Hence, the density of metal X is