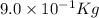Answer : The mass lost in this reaction will be,
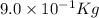
Explanation :
According to the Einstein equation, the energy is equal to the product of mass and the square of the speed of light.
The mathematical expression is :

where,
E = energy released =
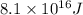
c = speed of light =
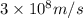
m = mass lost = ?
Now put all the given values in the above formula, we get the mass lost in the reaction.
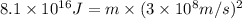
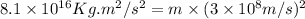

Therefore, the mass lost in this reaction will be,