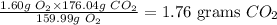Answer
1.76 grams CO2
Step-by-step explanation
Given data:
Reacting mass of oxygen = 1.60 grams
What to find:
Mass of carbon dioxide produced in grams.
Step-by-step solution:
The first step is to write the balanced chemical equation for the reaction.
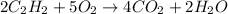
From the Periodic Table:
The Molar mass of CO2 = 44.01 g/mol
The Molar mass of O2 = 31.998 g/mol
So from the balanced chemical equation for the reaction;
5 mol O2 produced 4 mol CO2.
In grams, it implies
(5 mol x 31.998 g/mol) = 159.99 g O2 produced (4 mol x 44.01 g/mol) = 176.04 g CO2
Hence, 1.60 g O2 will produce: