Answer : The moles ratio between the nitrogen gas and hydrogen gas are, 1 : 3 respectively.
Explanation :
The balanced chemical reaction will be,
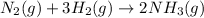
Mole ratio : It is defined as the ratio of the number of moles of the substances whose ratio is to be calculated.
In a chemical reaction, the stoichiometric coefficients represents the number of moles.
By stoichiometry of the reaction we can say that,
1 mole of nitrogen gas reacts with 3 moles of hydrogen gas to produce 2 moles of ammonia gas.
Hence, the mole ratio of between the nitrogen gas and hydrogen gas are, 1 : 3 respectively.