Answer:
33.16 g of fluorine (F2).
Step-by-step explanation:
What is given?
Mass of Cobalt (II) fluoride (CoF2) = 84.56 g.
Molar mass of CoF2 = 96.9 g/mol.
Molar mass of fluorine (F2) = 38 g/mol.
Step-by-step:
First, we have to state the chemical equation. Cobalt (II) chloride (CoCl2), and fluorine (F2) are the reactants, and the products are cobalt (II) fluoride (CoF2), and chlorine gas (Cl2):
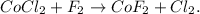
Our chemical equation is already balanced.
The next step is to calculate how many moles of CoF2 there are in 84.56 g of CoF2. To do this, we're going to use its molar mass:
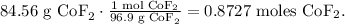
In the chemical equation, you can see that 1 mol of fluorine (F2) reacted to produce 1 mol of CoF2, so the molar ratio between them is 1:1. This means that we need 0.8727 moles of F2 to produce 0.08727 moles of CoF2.
So the final step would be to convert 0.8727 moles of F2 to grams using its molar mass, like this:
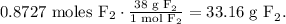
The answer is that we will need 33.16 g of fluorine (F2) to produce 84.56 g of cobalt (II) fluoride (CoF2).