Answer:
The percent yield is 77.18%.
Step-by-step explanation:
1st) According to the balanced reaction, 1 mole of Pb(NO3)2 reacts with 2 moles of KI to produce 2 moles of KNO3 and 1 mole of PbI2.
We can convert the moles to grams using the molar mass of Pb(NO3)2 (331.2g/mol), KI (166g/mol) and KNO3 (101g/mol):
- Pb(NO3)2 conversion: 331.2g
- KI conversion: 332g
- KNO3 conversion: 202g
2nd) Now, with the stoichiometry of the reaction in grams we can calculate the grams of KNO3 that must be produced (this is the Theoretical yield):
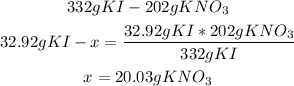
So, this is the theoretical amount of KNO3.
3rd) With the theoretical yield (20.03g) and the Actual yield (15.46g) we can calculate the percent tield:

So, the percent yield is 77.18%.