Answer:
17. 0.636 M of dilute solution.
18. 730 mL of H2O.
Step-by-step explanation:
17. Let's see the following formula:
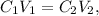
where 'C' represents concentration and 'V' the volume. Subindex 1 indicates compound 1 and subindex 2 indicates compound 2.
Compound 1 would be, in this case, the entire solution, and Compound 2 would be K2SO4. So, we want to know the value of 'C1'. Let's solve for C1 and replace the given data:
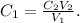
C2 would be 0.75 M, V2 would be 250 mL and V1 would be 295 mL because we're adding 45 mL to a 250 mL solution (45 mL+250 mL):
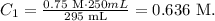
The molarity of the diluted solution would be 0.636 M.
18. To calculate the additional volume that we need of water, we use the initial formula. We can see that the compound 1 will be the final solution and compound 2 the solution of HCl, so we want to find V1:
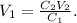
C2 is 37 M, V2 is 10 mL and C1 is 0.5 M:
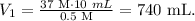
This would be the total volume of solution, so we have to find what is the amount of water that was added, like this:
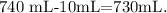
We subtract 10 mL because that was the volume of HCl. So we need 730 mL of H2O (water) to obtain a 0.5 M of solution from 37 M and 10 mL HCl.