1) Balance the chemical equation.
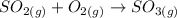
List the elements in the reactants.
S: 1
O: 4
List the elements in the products.
S: 1
O: 3
S is already balanced.
Balance O
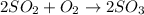
List the elements in the reactants.
S: 2
O: 6
List the elements in the products.
S: 2
O: 6
The balanced equation is
![2SO_2+O_2\operatorname{\rightarrow}2SO_3]()
2) Moles of SO3 formed from O2.
The molar ratio between SO3 and O2 is 2 mol SO3: 1 mol O2.
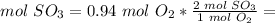
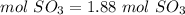
3) Convert moles of SO3 to molecules of SO3.
Avogadro's number is 6.022*10^23
1 mol SO3= 6.022*0^23 molecules of SO3.
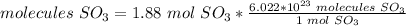
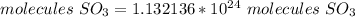
1.13*10^24 molecules are formed from 0.35 mol O2.
.