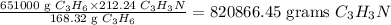Answer
820866.45 grams C₃H₃N can be obta
Step-by-step explanation
Given balanced equation:
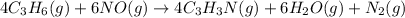
Given reacting mass of C₃H₆ = 651 kg
What to find:
The mass of C₃H₃N obtained from 651 kg of C₃H₆
Step-by-step soltion:
From the Periodic Table:
Molar mass of C₃H₆ = 42.08 g/mol
Molar mass of C₃H₃N = 53.06 g/mol
You need to convert the given reacting mass of C₃H₆ from kilograms, (kg) to grams, (g).
Conversion factor:
1 kg = 1000 g
So 651 kg = 651 x 1000 = 651,000 grams
From the balanced equation above;
(4 mol x 42.08 g/mol) = 168.32 g C₃H₆ produced (4 mol x 53.06 g/mol) = 212.24 g C₃H₃N
Therefore 651,000 g C₃H₆ will produce