Answer:
4.873g of AgCl will precipitate.
Step-by-step explanation:
1st) It is necessary to calculate the moles of magnesium chloride contained in 39.6mL of 0.428M solution:
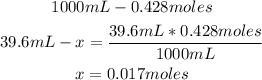
Now we know that there are 0.017moles of magnesium chloride in the solution.
2nd) According to the stoichiometry of the reaction, from 1 mole of magnesium chloride, 2 moles AgCl precipitate, so we can use a mathematical rule of three and the 0.017moles of MgCl2 to calculate the moles of AgCl that will precipitate:
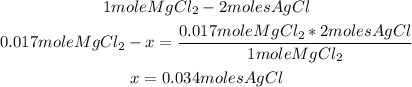
Now we know that 0.034 moles of AgCl are produced.
3rd) Finally, we can calculate the grams of silver chloride by using a mathematical rule of three with the molar mass of AgCl (143.32g/mol) and the 0.034moles:
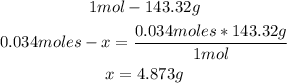
So, 4.873g of AgCl will precipitate.