2423Answer
Step-by-step explanation
Given:
Mass of copper = 154 kg
Convert kg to gram by multiplying by 1000
154 kg x 1000 = 154000 grams
From Avogadro's constat, 1 mole of any substance contains 6.022×10²³ atoms
The molar mass of copper, that is, the amount of mass that copper contains in one mole, is 63.546 g/mol. So, 154000 g of copper are contained in:
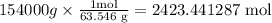
Since 1 mole of copper contains 6.022×10²³ atoms,
Therefore 2423.441287 moles of copper will contains: