ANSWER
The mass of H2SO4 is 2.06 grams
Step-by-step explanation
Given that;
The volume of Al(OH)3 is 60.0mL
The molarity of the solution is 0.233M
Follow the steps below to find the mass of H2SO4
Step 1; Write the balanced equation of the reaction
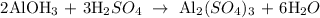
Step 2; Find the number of moles of Al(OH)3 using the below formula
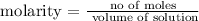
Convert the volume of the solution to liters
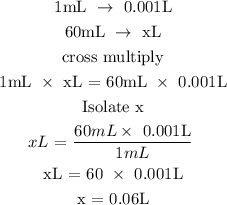
The volume of the solution in Liters is 0.06L
Substitute the given data into the formula in step 2

Therefore, the number of moles of Al(OH)3 is 0.01398 mol
Step 3; Find the number of moles of H2SO4 using a stoichiometry ratio
Let x represents the number of moles of H2SO4
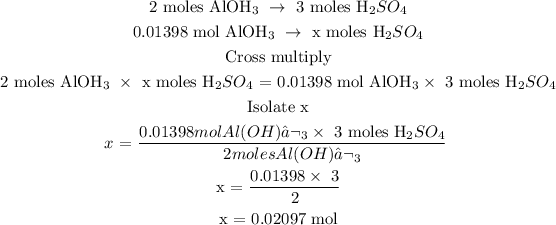
Therefore, the number of moles of H2SO4 is 0.02097 mol
Step 4; Find the mass of H2SO4 using the below formula
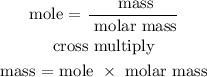
Recall, that the molar mass of H2SO4 is 98.09 g/mol
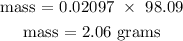
Hence, the mass of H2SO4 is 2.06 grams