ANSWER
The original volume of the gas is 4L
Step-by-step explanation
Given information
The initial pressure of the gas = 6.0atm
The final pressure of the gas = 0.5 atm
The final volume of the gas = 48L
Step 1: Write the Boyle's law formula
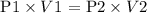
Where
P1 = 6.0 atm
P2 = 0.5 atm
V2 = 48L
V1 =?
Step 2: Substitute the given data into the formula in step 1
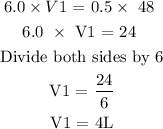
Hence, the original volume of the gas is 4L