We know that:
- number of moles of the gas: 4.1 mol
And we must calculate the volume as sample at STP.
We need to use that
Now, we must use the ideal gas equation

Since we need the volume we must solve the equation for V
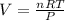
Finally, we must replace the values in the formula
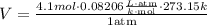
Simplifying,
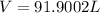
ANSWER:
Volume = 91.9002 L