Answer : The correct option is, (C) 6.3 mole Ag
Explanation : Given,
Number of atoms of Ag =

As we know that, 1 mole contains
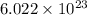 number of atoms.
number of atoms.
As,
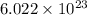 number Ag atoms present in 1 mole of Ag
number Ag atoms present in 1 mole of Ag
So,
 number Ag atoms present in
number Ag atoms present in
 mole of Ag
mole of Ag
Therefore, the number of moles of Ag present are, 6.3 moles