The average atomic mass is 185.4 amu.
To calculate the average atomic mass of an atom from its isotopes, it is necessary to multiply the mass by the percentage of each isotope, then sum them all, and finally, divide it by 100:
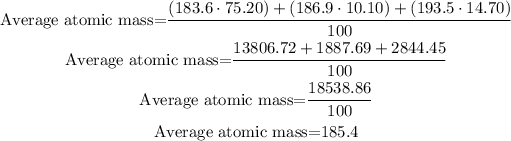
So, the average atomic mass of that element is 185.4 amu.