1) Write and balance the chemical equation
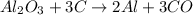
2) Calculate moles of aluminum oxide (Al2O3)
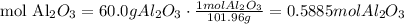
3) Calculate moles of carbon (C)
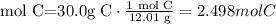
4) Find the limiting reactant
How many moles of Al2O3 do we need to use all of the C.
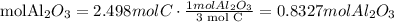
We need 0.8327mol Al2O3 and we have 0.5885 mol Al2O3. We do not have enough Al2O3. Al2O3 is the limiting reactant.
How many moles of C do we need to use all of the Al2O3.
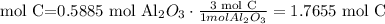
We need 1.7655 mol C and we have 2.498 mol C. We have enough C. This reactant is in excess.
5) Theoretical yield
The limiting reactant is Al2O3 (0.5885 mol)
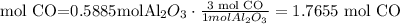
6) grams of CO produced.
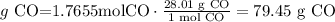
The theoretical yield of carbon monoxide is 49.01 g.
.