a. The first step to solve this part is to multiply the volume of solution times the concentration to find the moles needed:
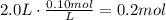
Then, multiply this amount of moles times the molecular weight to find the grams needed to prepare the solution:
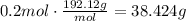
It means that 38.424 grams of citric acid are needed to make that solution.
b. To find the limit reagent, the first step is to convert the given amounts to moles.
Multiply the volume of solution times its concentration to find the amount of moles of citric acid. First convert the volume in mL to L:
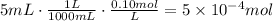
Divide 0.100g by the molecular weight of baking soda to find the moles of baking soda:
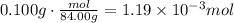
Now, divide each amount of moles by the corresponding coefficient in the reaction. It means, the amount of moles of citric acid divide it by 1 and the amount of moles of baking soda divide it by 3. Whichever result in a smaller number is the limiting reactant:
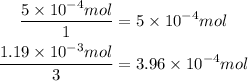
It means that the limit reagent is the baking soda.
c. The first step to solve this part is to find the amount of moles produced by the reaction, basing our calculations in the limit reagent and that the yield of the reaction is 90%.
According to the reaction, 3 moles of baking soda produce 3 moles of carbon dioxide, use the amount of baking soda to find the amount of carbon dioxide produced:
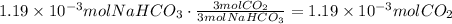
Now, multiply this by the yield of reaction to find the actual amount of CO2 produced:
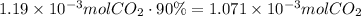
Use this amount of moles, the given pressure and temperature (convert it to kelvin degrees first) to find the volume of carbon dioxide using the ideal gas law.

Remember that R has a value of 0.082atmL/molK:
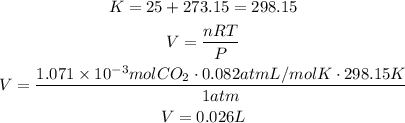
It means that 0.026L of carbon dioxide were collected.