1) List the known and unknown quantities.
Initial conditions:
Weight (pressure): 150 g.
Volume: 8.5 cc.
Final conditions:
Weight (pressure): 350 g.
Volume: unknown.
2) Assuming we can use weight as pressure, we can use Boyle's law.
This law states that there is a relationship between pressure and volume according to the following question:
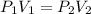
3) Plug in the known quantities and solve for V2.
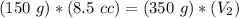
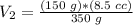
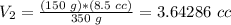
The volume in the syringe would be 3.6 cc if 350 g were used.
.