Answer:
Limiting reactant: lead(II) nitrate (Pb(NO3)2).
Mass of sodium nitrate (NaNO3) = 12.92 g.
Step-by-step explanation:
What is given?
Mass of lead (II) nitrate (Pb(NO3)2) = 25 g.
Mass of sodium iodide (NaI) = 30 g.
Molar mass of Pb(NO3)2 = 331 g/mol.
Molar mass of NaI = 150 g/mol.
Molar mass of sodium nitrate (NaNO3) = 85 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation. Lead (II) nitrate (Pb(NO3)2) reacts with sodium iodide (NaI) in a double-replacement reaction to produce sodium nitrate (NaNO3), and PbI2:
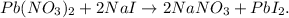
Now, let's calculate the number of moles of each reactant using its molar mass. The conversion from grams to moles for Pb(NO3)2 will look like this:
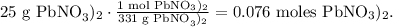
And for NaI:
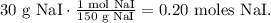
The next step is to see how many moles of NaNO3 are being produced. We're going to need the chemical equation: let's start with Pb(NO3)2. 1 mol of Pb(NO3)2 reacted produces 2 moles of NaNO3, so we will obtain:
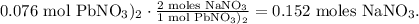
And now, let's see that 2 moles of NaI reacted produce 2 moles of NaNO3, so the molar ratio between these compounds is 1:1, which means that 0.20 moles of NaI reacted will produce 0.20 moles of NaNO3 too:
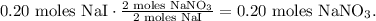
Based on these calculations, you can note that the limiting reactant would be Pb(NO3)2 because this compound imposes the limit because is being consumed first, it is producing the maximum amount of NaNO3 that we can produce in this reaction.
The final step is to calculate the mass of NaNO3 that is being produced. Remember as Pb(NO3)2 is the limiting reactant and it produces 0.152 moles of NaNO3, we use this data to find the mass of NaNO3 using its given molar mass too, like this:
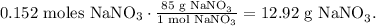
The answer is that the limiting reactant is lead (II) nitrate (Pb(NO3)2) and we're producing 12.92 g of sodium nitrate (NaNO3).