The formula of De Broglie is the following:
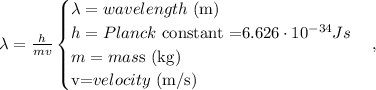
Remember that the mass of an electron is 9.11 x 10 ^(-31) kg, so replacing in the formula, we're going to obtain:
![\begin{gathered} \lambda=\frac{6.626\cdot10^(-34)J\cdot s}{9.11\cdot10^(-31)\operatorname{kg}\cdot1.48\cdot10^5(m)/(s)}, \\ \lambda=4.91\cdot10^(-9)\text{ m} \end{gathered}]()
The answer is that the wavelength of an electron traveling at 1.48 x 10^(5) m/s is 4.91 x 10^(-9) m.