From the question, it was given that the Uranium has a molar mass of 238 grams/mole. It means that for every mole of uranium, its mass will be 238 grams.
Because the question asks the mass of 3 moles of uranium, it will be 3 times the mass of a single mole of it. From this, we can perform the calculation as follows:
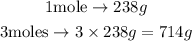
From the solution presented above, we are able to conclude that 3 moles of uranium weigh 714g.