Answer:
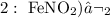
Step-by-step explanation:
Here, we want to get the incorrect formula for the Fe3+ ion
Now, this has to do with the ion of the counter-party
There has to be a proper exchange of charge
Now, let us look at the options:
In option 2, it is expected that the counter-party takes 3 as its subscript
Instead, it is taking 2, which corresponds to iron (ii)
In that case, we have the option as the correct choice of answer