Answer:
The amount of the substance is 14.6 g.
Step-by-step explanation:
Given that,
Energy = 350 J
Temperature
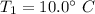
Temperature
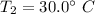
Specific heat capacity = 1.2 J/g^{/circ}[/tex]
We know that,
The formula of specific heat is defined as
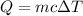
Where,
m = mass of the substance
c = specific heat capacity
 = Temperature change
= Temperature change
Put the value into the formula
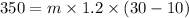
The amount of substance is
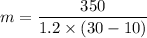
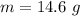
Hence, The amount of the substance is 14.6 g.