Answer:
a) 6.225 moles
b) 423.134grams
Explanations:
Given the balanced chemical equation as shown below:
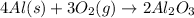
a) Given the following parameters
Moles of aluminium = 8.3moles
According to stoichiometry, 4 moles of aluminium reacted with 3 moles of oxygen, the moles of oxygen are required to react with 8.3 moles of aluminum is expressed as:
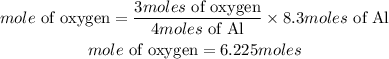
Hence the moles of oxygen required is 6.225moles
b) According to stoichiometry, 4 moles of Al produces 2 moles of aluminum oxide. The moles of aluminum oxide required will be:
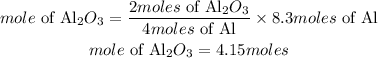
Determine the mass of the product
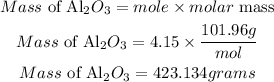
Hence the mass of product that will be formed from 8.3 moles of aluminum is 423.134grams