endpointVinegar is also called as acetic acid, and we can write it in the following way CH3COOH. When we talk about titration, it refers to the amount of base needed to neutralize the acid. So there is a chemical reaction involved. The reaction between vinegar and NaOH base is as follows:
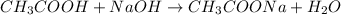
We have a 1 to 1 ratio, i.e. to neutralize x moles of vinegar we need the same moles of NaOH. Let's calculate how many moles of acid are believed to be in the solution. We will use the molarity = 0.78M = 0.78 mol/L.
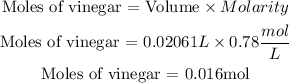
Then we need 0.016 moles of NaOH, the volume required will be:
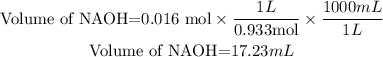
So, the volume that would be needed of NaOH to see an end point is 17.23 mL