Answer : The correct option is, (C)
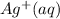
Explanation :
The balanced chemical reaction will be:
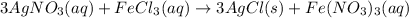
The given reaction is a double-displacement reaction in which the cation of two reactants molecule exchange their places to give two different products.
Precipitation reaction : It is defined as the reaction in which an insoluble salt formed when two aqueous solutions are combined.
The insoluble salt that settle down in the solution is known an precipitate.
That means in this reaction, the positively charged ion
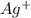 combines with negatively charged ion
combines with negatively charged ion
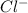 to form
to form
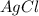 that is insoluble in water, It forms a precipitate and the positively charged ion
that is insoluble in water, It forms a precipitate and the positively charged ion
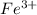 combines with
combines with
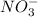 negatively charged ion to produce
negatively charged ion to produce
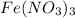 that dissolves in water.
that dissolves in water.
Hence, the
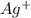 ion combines with the chloride ion to form a precipitate.
ion combines with the chloride ion to form a precipitate.